Are you hoping to find 'redox and metathesis reactions'? You can find all of the material on this webpage.
Double decomposition and redox reactions are chemical reactions where the products are completely divergent from reactants.Both reactions involve the central of something betwixt reactants to springiness the product (s). exchange of electrons, chemical moieties.These reactions involve two antonymous reactions. ...
Table of contents
- Redox and metathesis reactions in 2021
- 10 examples of redox reaction
- What is redox reaction
- When will reactions proceed to completion
- Redox reaction examples with answers
- Metathesis reaction calculator
- Metathesis reaction lab answers
- Redox reaction examples with answers pdf
Redox and metathesis reactions in 2021
 This image shows redox and metathesis reactions.
This image shows redox and metathesis reactions.
10 examples of redox reaction
 This image illustrates 10 examples of redox reaction.
This image illustrates 10 examples of redox reaction.
What is redox reaction
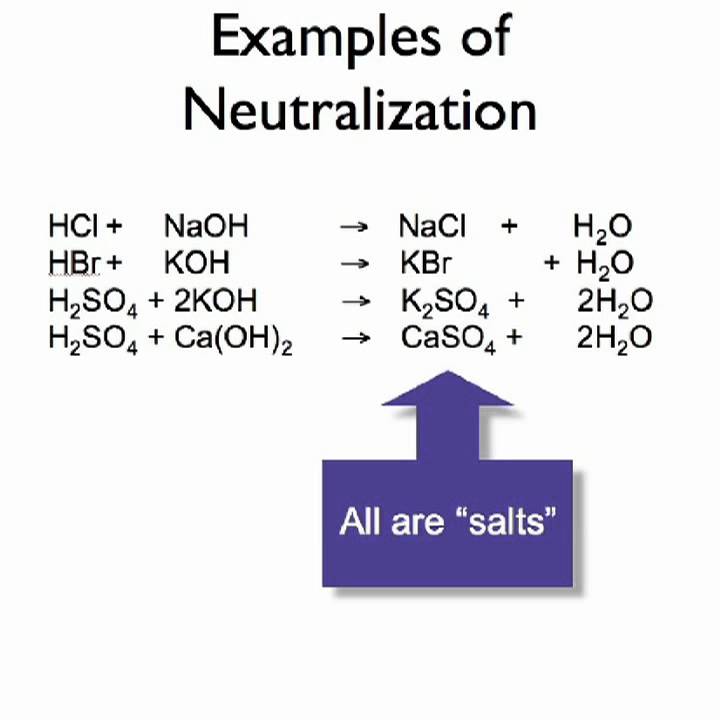 This image shows What is redox reaction.
This image shows What is redox reaction.
When will reactions proceed to completion
 This picture shows When will reactions proceed to completion.
This picture shows When will reactions proceed to completion.
Redox reaction examples with answers
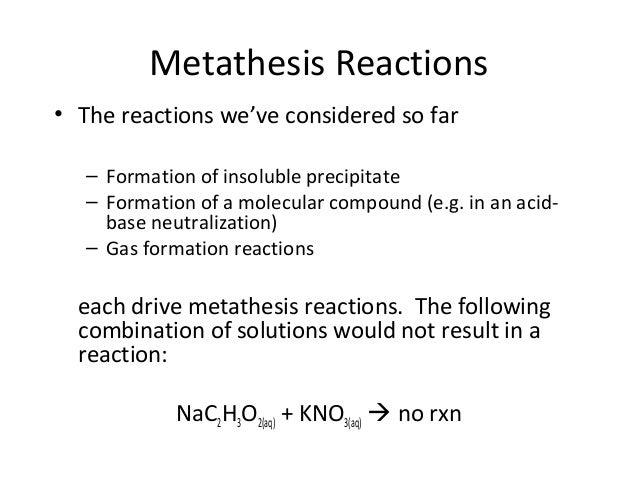 This image illustrates Redox reaction examples with answers.
This image illustrates Redox reaction examples with answers.
Metathesis reaction calculator
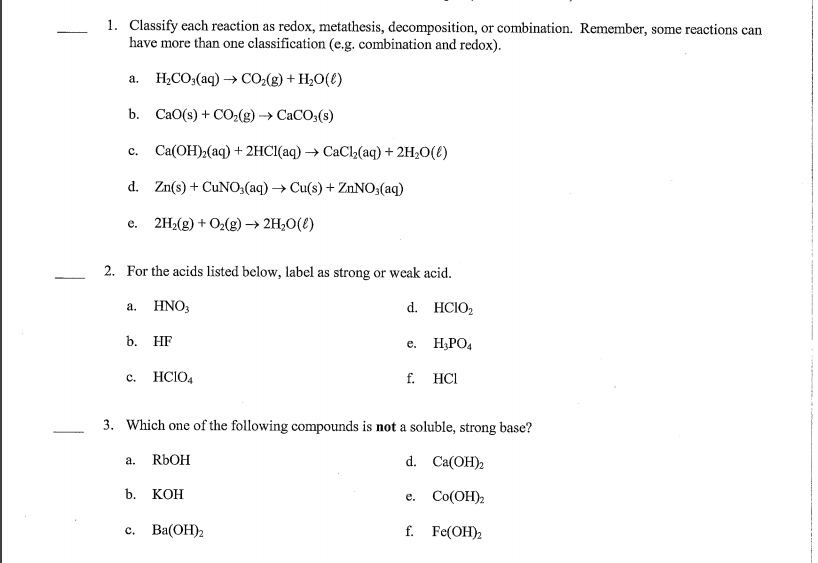 This picture demonstrates Metathesis reaction calculator.
This picture demonstrates Metathesis reaction calculator.
Metathesis reaction lab answers
 This image representes Metathesis reaction lab answers.
This image representes Metathesis reaction lab answers.
Redox reaction examples with answers pdf
 This picture demonstrates Redox reaction examples with answers pdf.
This picture demonstrates Redox reaction examples with answers pdf.
What's the difference between metathesis and redox reactions?
Metathesis and redox reactions are chemical reactions where the products are completely different from reactants. Both reactions involve the exchange of something between reactants to give the product (s). e.g exchange of electrons, chemical moieties. These reactions involve two complementary reactions.
Which is an example of a redox reaction?
In a redox reaction, the oxidation numbers of atoms are changed. Redox reactions may involve the transfer of electrons between chemical species. The reaction that occurs when In which I2 is reduced to I- and S2O32- (thiosulfate anion) is oxidized to S4O62- provides an example of a redox reaction: 2 S2O32−(aq) + I2(aq) → S4O62−(aq) + 2 I−(aq)
How is a double displacement reaction different from a redox reaction?
A double displacement reaction or metathesis is a single-step reaction, but a redox reaction has two parallel half-reactions required for the electron exchange process. Moreover, the oxidation states of atoms necessarily change during a redox reaction but, in metathesis reactions, it may or may not change.
Which is an example of a metathesis reaction?
What is Metathesis? 1 Precipitation reactions – A precipitate forms at the end of the reaction. For example, the reaction between silver... 2 Neutralization reactions – An acid neutralizes upon the reaction with a base. For example, an HCl solution (acid) can be... More ...
Last Update: Oct 2021