Are you looking for 'how to write ionic equations gcse'? All material can be found on this website.
Table of contents
- How to write ionic equations gcse in 2021
- Half ionic equations worksheet
- Balancing ionic equations worksheet with answers
- Ionic equations calculator
- How to balance ionic equations
- What is an ionic equation explain with an example
- Ionic equations a level
- Ionic equations worksheet and answers
How to write ionic equations gcse in 2021
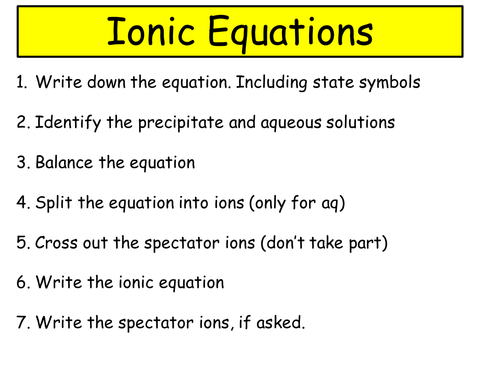 This image representes how to write ionic equations gcse.
This image representes how to write ionic equations gcse.
Half ionic equations worksheet
 This image demonstrates Half ionic equations worksheet.
This image demonstrates Half ionic equations worksheet.
Balancing ionic equations worksheet with answers
 This image demonstrates Balancing ionic equations worksheet with answers.
This image demonstrates Balancing ionic equations worksheet with answers.
Ionic equations calculator
 This image illustrates Ionic equations calculator.
This image illustrates Ionic equations calculator.
How to balance ionic equations
 This image demonstrates How to balance ionic equations.
This image demonstrates How to balance ionic equations.
What is an ionic equation explain with an example
 This picture shows What is an ionic equation explain with an example.
This picture shows What is an ionic equation explain with an example.
Ionic equations a level
 This picture illustrates Ionic equations a level.
This picture illustrates Ionic equations a level.
Ionic equations worksheet and answers
 This image shows Ionic equations worksheet and answers.
This image shows Ionic equations worksheet and answers.
How to write an ionic equation for neutralisation?
Ionic Equations for Neutralisation STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) ⟶ NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl – (aq) + Na (aq) +... STEP 3: Cancel out common ions, which are the spectator ions H ...
What are spectator ions in the ionic equation?
The Na+ ions and NO3- ions remain separate in the sodium nitrate solution and do not form a precipitate. Ions that remain essentially unchanged during a reaction are called spectator ions.This means you can ignore them when you write the ionic equation. You only need to model how the solid silver chloride forms:
How to write the ionic equation for nitric acid?
Write the ionic equation for the redox reaction between copper and nitric acid to form copper nitrate, nitrogen dioxide, and water. This is a tricky redox reaction. For every 4 nitrate ions involved, 2 nitrate ions take part in the reaction by oxidising copper to copper (II) ion. The other 2 nitrate ions are instead the spectator ions.
How do you write ionic equations in chemistry?
We can write ionic equations by starting from their chemical equations. Then, we rewrite the soluble ionic compounds as their dissociated ions. This is when you realise that some ions do not react: they remain dissociated in solution. These are the spectator ions, which we cancel out.
Last Update: Oct 2021
Leave a reply
Comments
Timberly
28.10.2021 01:244 write balanced geographical region equations - for displacement reaction. In this video i Am going to Edward Thatch you how to write ionic fractional equations which ar really useful stylish understanding electrolysis.
Ezequiel
26.10.2021 11:30Reciprocal ohm how to write out ionic equations gcse services, on the other hand, is a perfect catch for all my written needs.