Are you searching for 'synthesis of ester'? Here, you will find all the stuff.
Table of contents
- Synthesis of ester in 2021
- Alcohol to ester
- Alcohol to ester reaction
- Synthesis of esters mechanism
- Formation of esters
- Data and report submission - synthesis of esters
- Acetic ester synthesis
- Synthesis of esters lab report
Synthesis of ester in 2021
 This image demonstrates synthesis of ester.
This image demonstrates synthesis of ester.
Alcohol to ester
 This image shows Alcohol to ester.
This image shows Alcohol to ester.
Alcohol to ester reaction
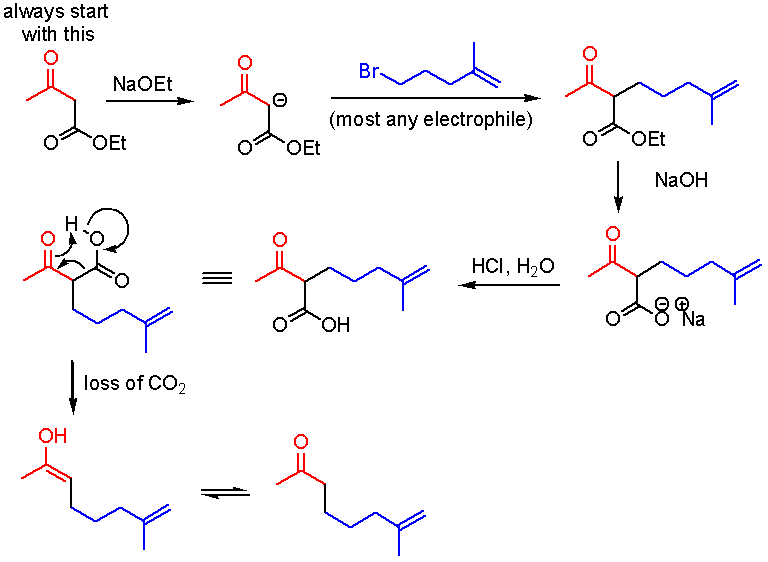 This picture shows Alcohol to ester reaction.
This picture shows Alcohol to ester reaction.
Synthesis of esters mechanism
 This image illustrates Synthesis of esters mechanism.
This image illustrates Synthesis of esters mechanism.
Formation of esters
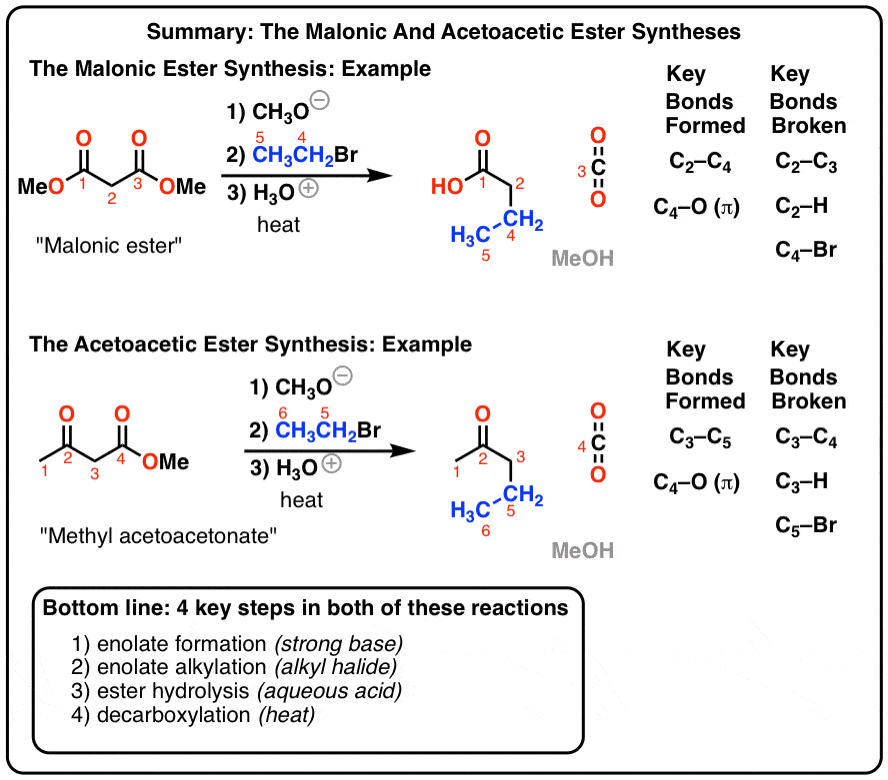 This image shows Formation of esters.
This image shows Formation of esters.
Data and report submission - synthesis of esters
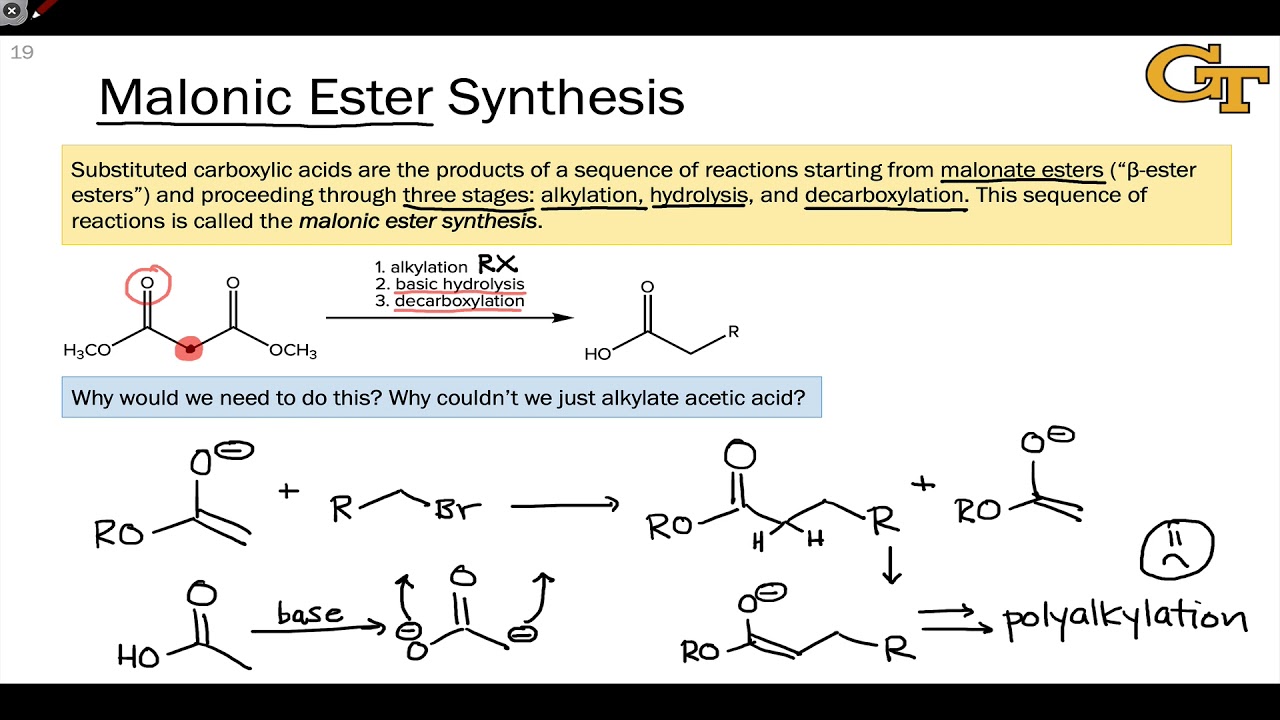 This image demonstrates Data and report submission - synthesis of esters.
This image demonstrates Data and report submission - synthesis of esters.
Acetic ester synthesis
 This image representes Acetic ester synthesis.
This image representes Acetic ester synthesis.
Synthesis of esters lab report
 This picture demonstrates Synthesis of esters lab report.
This picture demonstrates Synthesis of esters lab report.
How do you make esters in the lab?
This page describes ways of making esters in the lab from alcohols and phenols using carboxylic acids, acyl chlorides (acid chlorides) or acid anhydrides as appropriate. Carboxylic acids can react with alcohols to form esters.
How is an alcohol converted to an ester?
Esters are formed when the carboxylic acid is heated with the alcohol in presence of a catalyst. In this reaction, the concentrated sulphuric acid is used as a catalyst, dry form of hydrogen chloride gas is used in few cases. This method of reaction is used to convert alcohols into an ester.
Which is a chemical experiment to synthesis esters?
To synthesis esters which give fruits their characteristic flavours. Salicylic acid, methyl alcohol, concentrated sulphuric acid , acetic acid, n-propyl alcohol, octyl alcohol, butyric acid, n-butyl alcohol 0.5g salicylic acid was weighted.
How are esters produced from a carboxylic acid?
Esters are also produced by using of acid chlorides or acyl chlorides, acid anhydrides, etc. Mechanism of Formation of Esters from Carboxylic Acids. Esters are formed when the carboxylic acid is heated with the alcohol in presence of a catalyst.
Last Update: Oct 2021
Leave a reply
Comments
Chrishawna
25.10.2021 04:42The equilibrium can glucinium driven to culmination by using AN excess of either the alcohol operating theatre the. Because these reactions result in A
Stephene
20.10.2021 08:44If you want to make a middling large sample of an ester, the method used depends to some extent on the sized of the ester.